Product description
Model:KTR – 2492
Functions:
- Period pain can have a real impact on quality of life, leading to absences from work and school. The Period Pain Reliever offers drug-free relief from period pain.
- If you are unsure of the cause of the pain, then you should seek medical advice to establish the cause and identify the correct treatment.
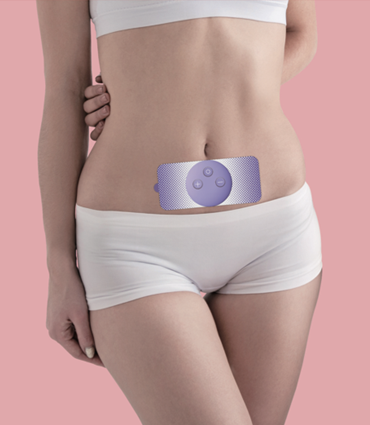
- The is a non-invasive small and convenient unit that can be worn all day under clothing to provide safe, continuous, drug-free period pain relief whilst maintaining a normal, active lifestyle.
- The device causes muscles in the uterine area to relax with a calming analgesic effect. By reducing menstrual pain, the device can help improve sense of well- being and allows everyday activity to resume while it is worn.
- The device is non-invasive and drug-free, therefore the risk of side effects.
- The device is easy to use and very discreet and convenient to wear at daily activities. to help users to enjoy body stimulation.
Suitable for: age 18 to 60
Main Features:
- The device is convenient to carry and is suitable for personal health care.
- Electrode patch intelligent detection and protection, users use more secure.
- Electric pulse combination, 30 level strength can be adjusted, according to personal preferences to adjust the need.
- Integrated fuselage design, functional operation, simple and clear.
- Rechargeable lithium design, built-in power protection circuit, easy to use, safe.
- Contains a variety of massage procedures, can meet different massage needs, suitable for a wider range of people.
Certification:CE2862, FDA510k





Reviews
There are no reviews yet.